Abstract
*DB and DK contributed to the work equally
Background
Prospective randomized controlled data comparing extracorporeal photopheresis (ECP) to other treatments for chronic graft vs host disease (cGvHD) as third-line or later therapy are limited, despite its clinical benefit observed in patients (pts) who failed ≥ 2 lines of previous therapy. Our single-center experience has reported promising results, including 68.3% failure-free survival (FFS) and 85.9% overall survival (OS) at 12 months in 75 heavily pre-treated cGvHD pts treated with ECP (ASH 2021 Abstract ID 152640).
The present study compared outcomes, using propensity-score matching (PSM), between ECP ("ECP group", n=74) and a historical cohort treated with best available therapy (BAT) as third-line or later treatment from 2007 to 2021 ("BAT group", n=132). Statistical endpoints such as FFS and OS, as well as steroid dose reduction were evaluated instead of overall response due to limited response assessment data available from retrospective chart review.
Patients and methods
The BAT group received MMF (n=71, 53.8%), prednisone (n=37, 28.0%), prednisone/cyclosporine (n=7, 5.3%), rituximab (n=7, 5.3%), and others (n=10, 7.6%). There was an imbalance in characteristics between the two groups, as expected; the ECP group had more pts with severe cGVHD (91.1% vs 20.5%; p<0.001), fewer with a previous history of acute GVHD (aGvHD: 60.8% vs 78.0%; p=0.008), and fewer on a prednisone dose ≥0.5mg/kg/day (37.8% vs. 90.5%; p<0.001). PSM analysis was applied to adjust risk factors imbalanced between groups, including cGVHD grade (mild/moderate vs severe), aGVHD history, and baseline prednisone dose (<0.5 vs. ≥ 0.5 mg/kg/day). A total of 54 pts (27 case-control pairs) were selected via PSM within 0.2 of a calliper difference, resulting in the balancing of risk factors between groups: cGVHD severity (p=0.941), aGVHD history (p=0.75) and prednisone dose ≥ 0.5 mg/kg/day (p=0.788).
FFS and OS were calculated from the day of starting ECP or BAT, and were compared using Cox's hazard model. Daily prednisone dose at months 0, 3 and 6 were calculated divided by body weight (kg), and the proportions of pts on prednisone ≤ 0, 0.1, 0.2 and 0.5mg/kg/day were compared.
Results
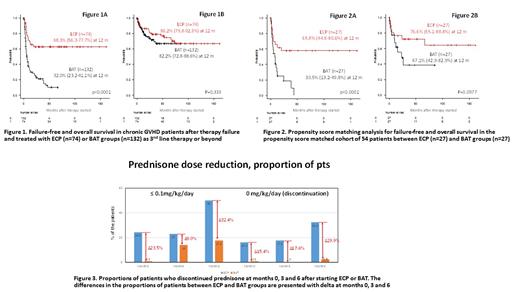
In the overall cohort (n=206), with a median 29 months of follow-up, 114 treatment failures (55.3%) occurred. While the non-relapse mortality (NRM) was similar in both groups, the ECP group showed a lower rate of resistance requiring therapy switch. Failure was noted in 27 ECP pts (36.4%) due to causes including resistance/intolerance requiring a switch to other therapy (n=15; 20.3%), NRM (n=11, 14.8%), and relapse (n=1; 1.4%), while 87 failures (65.9%) were noted in BAT pts due to resistance requiring a switch to other therapy (n=63; 47.7%), NRM (n=7; 5.3%), and relapse (n=17; 12.9%).
In the overall cohort, the 12-month FFS was 68.3% and 32.0% in ECP and BAT groups (p<0.0001; Fig 1A), while OS was 86.2% and 82.2% in ECP and BAT groups, respectively (p=0.333; Fig 1B). In the PSM cohort (n=54), the ECP group showed a survival benefit at 12 months: FFS was 65.8% in the ECP group vs. 30.5% in the BAT group (p=0.00226; Fig 2A), and OS was 76.6% in the ECP group vs. 67.1% in the BAT group (p=0.0977; Fig 2B).
Multivariate analysis in the PSM cohort confirmed that ECP was superior to BAT for FFS (p=0.024, HR 0.317 [0.117-0.859]) when adjusted for other risk factors including cGVHD severity, aGvHD history, age, HCT-CI score and prednisone dose ≤0.5mg/kg/day.
Prednisone doses were gradually reduced over time; the median doses of prednisone at months 0, 3, and 6 were 0.35, 0.22 and 0.11 mg/kg/day, respectively, in the ECP group vs. 0.96, 0.24 and 0.19mg/kg/day in the BAT group. ECP also showed better kinetics of steroid dose reduction over time; the proportions of pts who discontinued prednisone at months 0, 3 and 6 were 16.2, 17.6% and 32.4% in ECP group vs. 0.8%, 0% and 2.5% in BAT group (Fig 3). The differences in the proportion of pts (delta) who discontinued prednisone in the ECP vs. BAT groups were 15.4%, 17.6% and 29.9% at 0, 3, and 6 months, respectively.
Conclusion
In the current study using PSM analysis, use of ECP was associated with a superior FFS to BAT when used as third-line or later therapy in cGVHD patients who failed at least 2 lines of previous therapy. Use of ECP also allowed for better steroid tapering in comparison to BAT.
Patriquin: Alexion: Consultancy, Honoraria, Speakers Bureau; BioCryst Pharmaceuticals: Honoraria; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Apellis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria. Law: Novartis: Consultancy; Actinium Pharmaceuticals: Research Funding. Lipton: Bristol Myers Squibb, Ariad, Pfizer, Novartis: Consultancy, Research Funding. Mattsson: MattssonAB medical: Current Employment, Current holder of individual stocks in a privately-held company. Kim: Novartis: Consultancy, Honoraria, Research Funding; Paladin: Consultancy, Honoraria, Research Funding; Bristol-Meier Squibb: Research Funding; Pfizer: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal